Treatment of advanced gastrointestinal adenocarcinoma complicated with high-risk gastrointestinal stromal tumors (GISTs) in an elderly male patient: a case report and literature review
Introduction
Gastrointestinal adenocarcinoma is the most common malignant tumor in the digestive system, while gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumor. However, the coexistence of gastrointestinal cancer and GISTs is rare in patients (1,2), let alone advanced gastrointestinal adenocarcinoma with high-risk GISTs. With the research continued to conduct in recent years, GISTs associated with other tumors have attracted the attention of relevant researchers (2-4). However, there is very little literature exploring the adjuvant therapy strategies for synchronous gastrointestinal adenocarcinoma and GISTs. We report an elderly patient with synchronous advanced colorectal cancer (CRC) and high-risk GISTs and discuss the related drug therapy from a systematic review of the literature.
Case presentation
A 79-year-old male presented with a history of abdominal pain and diarrhea for a month as well as stopped venting and defecating for nine days.
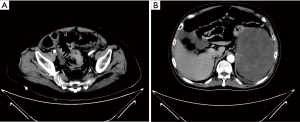
CT scan showed the segmental thickening of the distal part of sigmoid colon wall and stricture of the intestinal cavity, considering as the combination of sigmoid colon cancer with low intestinal obstruction (Figure 1A). The large space-occupying lesions in the left upper abdomen and spleen and stomach gap are closely related to the gastric wall, considering the possibility of interstitial tumor, not completely excepting for metastatic tumor or mesenchymal tumor invasion of gastric wall (Figure 1B).
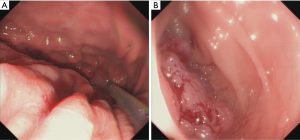
The patient underwent gastroscope in our hospital, which showed deformation and stenosis of fundus of stomach and body of stomach (Figure 2A), considering compressive lesion of gastric wall. Combined with CT, we considered exogenous mesenchymal tumors. Colonoscopy showed sigmoid colon cancer with obstruction, considering severe edema of the bowel wall, so the stent was placed for decompression (Figure 2B).
Subsequently, the patient underwent radical sigmoid colon resection, gastric neoplasm resection combined with splenectomy and permanent transverse colostomy on June 28th, 2018. Histopathological examination reveals:
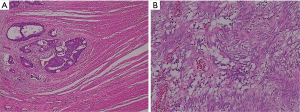
- A moderately differentiated adenocarcinoma in the sigmoid colon, infiltrating into subserosa (Figure 3A).
- LN1 and LN2 lymph nodes show metastasis (5/22, 3/7), while LN3 lymph nodes show no metastasis (Figure 3B).
- Splenic and abdominal masses (about 15.3 cm × 10.2 cm × 4.8 cm) suggest a high risk of GISTs.
- Mitotic count ≥5/50 high power fields (HPF).
- Immunohistochemical results: actin(+), CD34(+), CD117(+), Dog-1(+), Ki-67(+) (<1%), desmin(−) and S-100(−).
Consequently, the patient was finally diagnosed with advanced sigmoid colon cancer staging IIIB (pT3N1M0) (5) according to the NCCN clinical practice guidelines in Oncology and gastric GISTs with high-risk according to the modified NIH criteria (6).
Given that this elderly patient is poor tolerance to systemic vein chemotherapy for advanced CRC, we gave him oral chemotherapy regimen of capecitabine (1.5 g ×2 daily on days 1–14 every 21 days).Additionally, to prevent the recurrence, this patient was also received imatinib (400 mg ×1 daily). After 2 months follow-up, no adverse drug reactions were observed, and no recurrence was found in abdominal CT. The case provides a possibility of the capecitabine combined with imatinib for the elderly patient with synchronous advanced CRC and high-risk GISTs after the curative resection of tumor.
Discussion
The coexistence of advanced gastrointestinal adenocarcinoma with high-risk GISTs in a patient is rare, neither does the treatment experience used for reference. In 2010, Kumar et al. (7) reported two cases of coexistence of these two tumor types. The patient of the first case, diagnosed with metastatic GISTs, took 400 mg of imatinib mesylate and found remission in both the primary and liver lesions. Eleven months later from the starting of receiving imatinib mesylate, the descending colon lesions with lymph node metastasis were identified in the patient. Then the left hemicolon and empty field GISTs resection were performed for her. Subsequently, she received modified FOLFOX-6 chemotherapy and imatinib without any unexpected toxicity from the adjuvant therapy and remains well with continued regression of her liver metastasis (GISTs). The second case, a 61-year-old male, also took adjuvant FOLFOX chemotherapy and imatinib (400 mg per day) after colon surgery that confirmed the synchronous presence of GISTs. The report suggests that the concurrent treatment of FOLFOX chemotherapy and adjuvant imatinib is safe and effective for the patients with coexistence of GISTs and gastrointestinal adenocarcinoma. However, the current guidelines couldn’t provide us with relevant information about the safety for an aged patient.
Thus, we further searched the literature for detailed records of the specific doses, intervals and toxicity of the combination regimens. Halperin et al. (8) studied 20 patients with the endocrine cancers and proposed the recommended phase II regimen, which is dacarbazine 250 mg/m2 daily on day 1–3, capecitabine 500 mg/m2 twice daily on days 1–14, and imatinib 300 mg daily on days 1–21 of a 21-day cycle. Assessment results show that the most common toxicities were fatigue and edema, each occurring in 65% of patients. The most common grade 3 adverse event was dyspnea, with 30% of patients describing that symptom. Most treatment-related adverse effects were transient, and only one patient required dose reduction. All that illustrates the regimen of imatinib and capecitabine is well-tolerated.
It happens that there are two similar cases that Mayr’s (9) and Hoehler’s (10) reported manifesting targeted inhibition of platelet derived growth factor receptor (PDGFR) by imatinib could influence tumor growth and amplify chemotherapeutic effects. What’s more, making a combination of imatinib with other medication is tolerable and has promising efficacy. Although these cases are not gastrointestinal adenocarcinoma patients with GISTs, the individualized diagnosis and treatment of such patients should be worth our attention in the era of accurate therapy. Furthermore, more share and exploration in the discussion session are expected to be reported to advance relevant research.
Based on the evidence in the above literatures, it is safe and feasible for advanced CRC and high-risk GISTs to be treated with systemic intravenous chemotherapy and imatinib. In view of this elderly patient with 79 years old instead of systemic intravenous chemotherapy, he was given oral chemotherapy combined with imatinib. No adverse drug reactions were observed during the 2-month follow-up period. We will continue to track the patient and observe the safety of the combination. In the end, although there have been few reports on patients with advanced CRC complicated with high-risk GISTs, these cases might provide important and valuable references for the formulation of drug regimens.
Conclusions
The case with advanced gastrointestinal adenocarcinoma complicated with high-risk GISTs is rather rare, and there are no accurate references or guidelines for its treatments. We preliminarily came to the conclusion that the combination of imatinib and chemotherapy has certain clinical significance in the treatment of advanced gastrointestinal adenocarcinoma with high-risk GISTs. Our patient in this case report is suitable to receive imatinib and capecitabine, and the specific doses and intervals of the regimen need to be further explored with more cases.
Acknowledgments
Funding: This study was supported by the State’s Key Project of Research and Development Plan (2017YFC0108300 and 2017YFC0108303), and Academic Education Research Project of Nanfang Hospital.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gist.2019.01.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Liu S, Liu H, Dong Y, et al. Gastric carcinoma with a gastrointestinal stromal tumor - A case report and literature review. Med Sci (Paris) 2018;34 Focus issue F1:15-9.
- Fernandez JA, Olivares V, Gomez-Ruiz AJ, et al. Additional malignancies in patients with gastrointestinal stromal tumors (GIST): incidence, pathology and prognosis according to a time of occurrence-based classification. Clin Transl Oncol 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Hechtman JF, DeMatteo R, Nafa K, et al. Additional Primary Malignancies in Patients with Gastrointestinal Stromal Tumor (GIST): A Clinicopathologic Study of 260 Patients with Molecular Analysis and Review of the Literature. Ann Surg Oncol 2015;22:2633-9. [Crossref] [PubMed]
- Fernandez Hernandez JA, Olivares Ripoll V, Parrilla Paricio P. Additional primary malignancies in patients with gastrointestinal stromal tumors. Proposal for a new classification. Med Clin (Barc) 2016;147:405-9. [PubMed]
- Benson AB, Venook AP, Cederquist L, et al. Colon Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:370-98. [Crossref] [PubMed]
- Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol 2008;39:1411-9. [Crossref] [PubMed]
- Kumar K, Rowsell C, Law C, et al. Coexistence of gastrointestinal stromal tumour and colorectal adenocarcinoma: Two case reports. J Gastrointest Oncol 2011;2:50-4. [PubMed]
- Halperin DM, Phan AT, Hoff AO, et al. A phase I study of imatinib, dacarbazine, and capecitabine in advanced endocrine cancers. BMC Cancer 2014;14:561. [Crossref] [PubMed]
- Mayr M, Becker K, Schulte N, et al. Phase I study of imatinib, cisplatin and 5-fluoruracil or capecitabine in advanced esophageal and gastric adenocarcinoma. BMC Cancer 2012;12:587. [Crossref] [PubMed]
- Hoehler T, von Wichert G, Schimanski C, et al. Phase I/II trial of capecitabine and oxaliplatin in combination with bevacizumab and imatinib in patients with metastatic colorectal cancer: AIO KRK 0205. Br J Cancer 2013;109:1408-13. [Crossref] [PubMed]
Cite this article as: Chen L, Chen T. Treatment of advanced gastrointestinal adenocarcinoma complicated with high-risk gastrointestinal stromal tumors (GISTs) in an elderly male patient: a case report and literature review. Gastrointest Stromal Tumor 2019;2:1.