
New molecularly targeted drugs for gist after imatinib, sunitinib and regorafenib: a narrative review
Introduction
Gastrointestinal stromal tumors (GISTs) are mesenchymal neoplasms that arise from the gastrointestinal tract (1).
GISTs are thought to originate from the Cajal interstitial cells (2), which are pacemaker cells responsible for intestinal peristaltic contractions and are found in the myenteric plexus of the gastrointestinal tract. These tumors can occur anywhere along the gastrointestinal tract.
GIST are most usually seen in the stomach (50–60%) and the small bowel (30–35%), with the colon and rectum (5%) and esophagus (1%), being the least prevalent (3).
They account for roughly 20% of soft tissue sarcomas with an annual incidence of approximately 10 per million people.
They can anyone of any age, but more than 80% of those affected are over 50 years old (with a median age of 60–65 years) (4).
GIST is generally linked with a syndrome (Carney’s triad, Carney-Stratakis syndrome, and type 1 neurofibromatosis) in patients younger than 20 years (approximately 0.4 percent) (5-7).
Biological background
There are three histological patterns, morphologically different from each other, recognized in GIST: spindle cell, epithelioid and mixed (8).
GISTs have two particularly sensitive and specific histological markers: KIT (also known as CD117; present 95%) and Anoctamin1 (ANO1, also known as DOG1; present in 98%) (9,10).
Only 5% GISTs are negative for KIT, but the expression of ANO1 is present in many of these cases. This molecular expression is important because it will allow the response to KIT-targeted treatment even in the KIT-negative GIST subset (11).
Activating mutations in KIT and PDGFRA (which encode KIT and platelet-derived growth factor receptor tyrosine kinases, respectively) are currently thought to be the principal oncogenic drivers of GIST (2). Micro-GISTs (less than 1 cm) have similar mutations to clinical GISTs, implying that more genetic abnormalities are necessary for tumor growth.
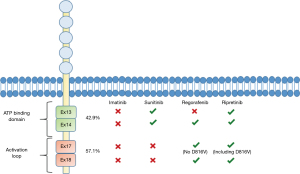
KIT mutations are found in 75–80% of GISTs. Exon 11 encodes the juxtamembrane domain, which is most affected by these alterations (90%). Deletions, frame insertions, missense mutations, and combinations are all examples of molecular changes. KIT’s extracellular domains (typically exon 9; prevalence about 8%) and kinase domains I and II (exons 13 and 17; prevalence about 2%) are also mutated, but in a smaller percentage of instances (12).
PDGFRA mutations account for 10–20% of GIST mutations, particularly in exons 12, 14, and 18 (the KIT and PDGFRA mutations are mutually exclusive) (13). KIT and PDGFRA kinase domains are generally activated by ligand binding (stem-cell factor or platelet-derived growth factor), resulting in receptor dimerization (14). These kinases’ juxtamembrane regions control dimerization, and mutations in these domains affect this function (15). Changes in the kinase II domains of KIT and PDGFRA, on the other hand, change the activation loop that controls the ATP-binding pocket conformation of each kinase. KIT and PDGFRA mutations increase oncogenic signaling via the mitogen-activated protein kinase (MAPK) and phosphoinositide-3-kinase (PI3K) pathways through these and perhaps other mechanisms (16). About 5–10% of GISTs, referred to as wild-type, lack either a KIT mutation or a PDGFRA mutation. Despite the name, neurofibromatosis 1 (NF1 gene mutation), Carney-Stratakis syndrome (rare), Carney triad (rare), BRAF mutation (rare), succinate dehydrogenase (SDH) subunit mutations (SDHA, SDHB, SDHC, SDHD), and RAS-family mutations (HRAS, NRAS, KRAS) are now known to be present in this subtype of GIST (17,18). For the optimal treatment of GISTs, the mutational analysis of KIT and PDGFRA is mandatory. Approximately 8% of GISTs have a PDGFRA D842V mutation, which provides primary resistance to imatinib and other approved tyrosine kinase inhibitors (TKIs) (19); however, most of them can respond to avapritinib (20).
Treatment for metastatic GIST: imatinib, sunitinib and regorafenib
Before the advent of TKIs, chemotherapy was the first line of therapy for metastatic GISTs, with unsatisfactory responses and a median survival of approximately 1 year (21). In the first phase I study, imatinib was tested at various doses, ranging from 400 mg per day to 500 mg twice a day; the 400 mg twice daily dose was established as the maximum tolerated dose (MTD) (22). In phase I and II studies where imatinib was administered at a daily dose of 400–600 mg, response rates [complete (CR) and partial (PR)] were between 40% and 74%, values similar to those obtained with the dosage of 800 mg per day (between 45% and 52%) but with a lower toxicity (22,23).
The efficacy of imatinib at 400 and 800 mg per day was compared in the pivotal phase III studies 6200550.51 and S003352. Both studies found that using imatinib 400 mg once a day had a significant therapeutic benefit. CR rates were between 3% and 6%, PR rates were between 45% and 48%, and disease stability rates (SD) were between 26% and 32%. Because there was no difference in overall survival (OS) between the two dosages, the 400 mg once day dose was designated as the standard dose. OS was 47 to 55 months, which was a significant improvement over chemotherapy. The imatinib 800 mg daily arm had a better progression-free survival (PFS) in a joint analysis of trials S0033 and the European Organization for Research and Treatment of Cancer 62005 (24). KIT exon 9 mutant tumors treated at the greatest dose showed improvement (25). Patients with exon 9 mutations should get imatinib 800 mg daily if tolerated, in light of this. The adverse effects of therapy at larger dosages are reduced when imatinib is started at 400 mg once day and gradually increased to the goal dose of 800 mg daily (26).
In advanced GISTs with a progression disease during therapy with imatinib 400 mg per day, imatinib dose escalation at 600 or 800 mg per day may lead to disease stability in approximately one third of patients and a response in 2% of patients. After dose escalation, over two-thirds of patients who respond or have stable illness with imatinib 800 mg stay progression-free for more than 2 years (27).
Sunitinib is a multitargeted TKI that inhibits KIT, PDGFR, vascular endothelial growth factor receptor (VEGFR), and FLT-1/KDR. MTD was determined in phase I investigations to be 50 mg per day for 28 days with 14 days of rest (28). This medicine is used as a second-line treatment following imatinib progression or as a first-line treatment in individuals who are unable to take imatinib. In 312 patients, a phase III research compared the use of placebo vs. sunitinib (50 mg per day, orally, in 6-week cycles with 4 weeks of activation and 2 weeks of interruption). The median duration to disease progression was 6.3 months with sunitinib and 1.5 months with placebo in this research [hazard ratio (HR) 0.33, 95% confidence interval (CI): 0.23–0.47; P=0.001]. Fatigue, diarrhea, skin discolouration, and nausea were the most common adverse effects (29). Patients with a KIT exon 9 mutation or wild type (WT) genotype showed a better PFS and survival rate than those with a KIT exon 11 mutation (30).
Regorafenib is a VEGFR1–3, TEK, KIT, RET, RAF1, BRAF, PDGFR, and FGFR multitarget TKI. This medicine is licensed for the treatment of GIST patients who have previously received imatinib or sunitinib (31). Regorafenib is given at a dose of 160 mg per day for 21 days, followed by a 7-day break, and repeated every 28 days. The GIST-Regorafenib in Progressive Disease (GRID) study, was a phase III, randomized, placebo-controlled trial in which patients were switched to regorafenib when their disease progressed with placebo. Regorafenib patients had a median PFS (mPFS) of 4.8 months compared to 0.9 months for placebo patients (HR 0.27, 95% CI: 0.18–0.39; P=0.001) (32).
Secondary resistance
Despite the great majority of patients with metastatic GIST showing a durable benefit from imatinib and the recent identification of long-term survivors with sustained responses (<2 years) (33), most of them develop imatinib resistance and progress within 2 years of treatment. Between 46% and 67% of patients develop secondary drug-resistance mutations due to TKI exposure (34,35). Increasing evidence suggests that each TKI has its own resistance profile. The most frequent mechanism of acquired resistance to imatinib is the occurrence of subclones harboring secondary KIT point mutations. These mutations usually involve exons 13 and 14, encoding for KIT ATP-binding domain, and/or exons 17 and 18, encoding for the activation loop and resulting in the stabilization of KIT in the active conformation, so preventing imatinib binding (36) (Figure 1). Several mutations can coexist in the same disease, reflecting inter- and intra-lesional heterogeneity of molecular drug resistance mechanisms in progressing GIST (35). After the onset of imatinib resistance, KIT secondary mutations can be detected through the analysis of circulating tumor DNA (ctDNA), which can be considered a useful and non-invasive method for the selection of targeted agents and predictions of antitumor effects (37). Despite most resistant tumors remaining addicted to the initial driver oncogene, alternative mechanisms for drug failure have been proposed, particularly in GIST lacking KIT mutations (WT). These resistance mechanisms include MAPK pathway activation, IGFR1 or AXL amplification, upregulation of focal adhesion kinase (FAK) and AKT or intratumoral VEGF expression (38,39). Several novel therapeutic strategies are being developed. Currently under investigation there are new next-generation selective tyrosine kinases, inhibiting a broader spectrum of secondary mutations or with a target-specific secondary KIT mutation.
Methods
An analytical and comparative PubMed research for novel therapeutic strategies in GIST treatment was conducted. All types of articles with a focus on prospective randomized trials and large meta-analysis were included. The search period has been from the year 2008 till 2021 to guarantee more recent studies on this topic. We used the following keywords: “GIST”, “Avapritinib”, “Ripretinib”, “target therapy”, “kinase inhibitors” and “combination”. A total of 637 items were identified. After removing duplicates and screening titles and abstract, 376 full text papers were evaluated. In total, 279 papers were further eliminated thus 97 relevant articles were considered eligible (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search (specified to date, month and year) | 10/Feb/2021–30/Jun/2021 |
| Databases and other sources searched | PubMed |
| Search terms used | “GIST”, “Avapritinib”, “Ripretinib”, “target therapy”, “kinase inhibitors” and “combination” |
| Timeframe | 2008–2021 |
| Inclusion and exclusion criteria | Randomized trials and large meta-analysis, English language |
| Selection process | All authors, removed duplicates, screening titles and abstract |
GIST, gastrointestinal stromal tumor.
Novel drugs
Traditional TKIs (imatinib, sunitinib and regorafenib) have revolutionized GIST treatment but the development of secondary resistance has become one of the major challenges in the management of locally advanced and metastatic GISTs.
After the approval of regorafenib in 2013 as a third-line therapy, several efforts have been made to find novel therapies capable of targeting the broad range of KIT and/or PDGFRA secondary mutations arising in individual patients (40) and latterly new molecularly targeted drugs have shown encouraging results in patients whose GIST is resistant to the conventional treatment options (41) (Table 2).
Table 2
| Drug | Line | Phase | N | Comparator | RR | PFS | OS | AE |
|---|---|---|---|---|---|---|---|---|
| Ripretinib | 4th | III | 129 | Placebo | 9.4% | 6.3 months | 15.1 months | Alopecia, fatigue, nausea, myalgia |
| Avapritinib, PDGFRA D842V | Any | I | 56 | Single arm | 88% | N/A | N/A | Memory impairment, hyperbilirubinemia, hypertension |
| Sorafenib | 3rd | II | 38 | Single arm | PR 13%, SD 55% | 5.2 months | 11.6 months | Hand and foot syndrome, hypertension, diarrhea |
| Pazopanib | 3rd | II | 81 | BSC | SD 84% | 3.4 months | 17,4 months | Hypertension, pulmonary embolism |
| Nilotinib | 3rd | III | 248 | BSC | CBR 52.7% | 3.6 months | 13.3 months | Nausea, abdominal pain, fatigue |
| Cabozantinib | 3rd | II | 50 | Single arm | PR 14%, SD 68% | 5.5 months | 18.2 months | Diarrhea, palmoplantar erythrodysesthesia, fatigue |
| Dovitinib | 2nd | II | 39 | Single arm | PR 2.6%, SD 50% | 4.6 months | Not reached | Hypertension, fatigue, vomiting |
| Masitinib | 2nd | II | 44 | Sunitinib | 20% | 3.7 months | 29.8 months | Rash, neutropenia |
| Ponatinib | 2nd or 4th | II | 39 | Single arm | CBR 35% | 2.8 months | N/A | Pain, hypertension, γ-GT or lipase increasing |
| Dasatinib | 3rd | II | 47 | Single arm | PR 32%, SD 24% | 2 months, 8.4 months WT GIST | 19 months | Constitutional pain, myelosuppression |
| Vatalanib | 2nd | II | 45 | Single arm | PR 4.4%, SD 35.6% | 4.5 months | N/A | Hypertension, nausea, dizziness, proteinuria |
| Linsitinib, wild type GIST | Any | II | 20 | Single arm | CBR 40%, PMR 12% | 52% at 9 months | 80% at 9 months | Nausea, fatigue, elevated liver function test |
AE, adverse events; BSC, best supportive care; CBR, clinical benefit rate; GIST, gastrointestinal stromal tumor; N, number of patients; N/A, not available; OS, overall survival; PFS, progression-free survival; PMR, partial metabolic response; PR, partial response; RR, response rate; SD, stable disease; WT, wild type; γ-GT, γ-glutamyl transpeptidase.
The main objective of this review is to provide an overview of the newest drugs developed for the management of metastatic GIST and to discuss new candidate targets on the horizon that can cover conventional TKIs secondary resistance mutations and expand the treatment landscapes of GISTs.
This review has few limitations. First, there were only a few randomized studies included. Some of the articles are retrospective in nature, which may have led to selection and reporting bias. Therefore, heterogeneity may exist among the selected randomized clinical trials due to the study protocols, patient baseline characteristics, and response evaluation bias. We present the following article in accordance with the Narrative Review reporting checklist (available at https://gist.amegroups.com/article/view/10.21037/gist-21-6/rc).
Ripretinib
Ripretinib (also known as DCC-2618) is a type II tyrosine switch control kinase inhibitor that has been shown to inhibit KIT and PDGFRA kinase signaling through a novel double mechanism of action: it binds precisely and strongly to both the activation loop and the switch pocket to seal and stabilize the kinase in the inactive or off state, arresting downstream signaling and cell proliferation (42). Ripretinib in vitro showed powerful antineoplastic effects thanks to its ability to bind with high affinity to KIT receptors with mutations in exons 9,11,13,14,17,18 and PDGFRA receptors with 12,14 and 18 mutated exons (43). Ripretinib also demonstrated to inhibit other receptors such as platelet-derived growth factor receptor beta (PDGFRB), VEGFR2, BRAF and TIE2 (angiopoietin-1 receptor) (44).
In 2015 a phase I study (45) evaluated dose-limiting toxicities (DLTs), MTD, safety and antitumor activity in 258 patients, including 184 patients with advanced GIST, with intolerance to or experiencing progression after one or more line of treatment and 74 patients with other neoplasms with amplification and/or mutations determining sensitivity to ripretinib.
In the dose-escalation section of the study patients (n=68) were given ripretinib 20–200 mg twice daily or 100–250 mg once daily in consecutive 28-day cycles until disease progression, study discontinuation or intolerable toxicity.
No MTD was reached as <33% of patients experienced a DLT at every dose level.
The study led to the recommended phase II dose (RP2D) of 150 mg once daily taken orally, which was related with an agreeable tolerability and safety profile.
Ripretinib was generally well tolerated and only 5.6% of patients dropped out because of treatment-emergent adverse event (TEAE).
One of the most common TEAE was grade 1 alopecia (62%) whose pathogenesis is still undefined, but perhaps due to inhibition of several other kinases besides KIT and PDGFRA.
Other toxicities were mostly manageable, like palmar-plantar erythrodysesthesia (43.7%), reported with grade 3 in only one patient (0.7%), fatigue (54.9%), myalgia (48.6%), nausea (45.8%), decreased appetite (33.8%) and diarrhea (33.1%).
Grade 3 or 4 lipase elevation was described in 17.6% of patients but was generally asymptomatic and not clinically relevant, while in two patients pancreatitis was diagnosed but with improvement after a dosing interruption and no recurrence after restarting treatment.
Early antitumor activity results in patients with GIST taking ripretinib showed encouraging efficacy among all line of therapy: objective response rate (ORR) and median progression free survival were respectively 19.4% and 10.7 months in 31 patients in second line, 14.3% and 8.3 months in 28 patients in third line and 7.2% and 5.5 months in 83 patients in fourth line or beyond.
Preliminary results of this study contributed to the design of INVICTUS (NCT03353753) (38), a double-blind, randomized, placebo-controlled phase III trial of ripretinib in previously treated patients with advanced GIST.
A total of 129 patients were randomized in a 2:1 ratio to receive either oral ripretinib 150 mg once daily (n=85) or placebo (n=44), allowing cross over to ripretinib in case of disease progression.
The trial achieved its primary endpoint, as in the double-blind period mPFS of patients taking ripretinib was 6.3 months (95% CI: 4.6–6.9 months) compared with 1.0 months of those receiving placebo (95% CI: 0.9–1.7 months) with a HR of 0.15 (95% CI: 0.09–0.25, P<0.0001).
Since the ORR was not statistically significant, due to hierarchical testing median OS was not formally tested, but in the experimental arm mOS was 15.1 months (95% CI: 12.3–15.1 months) and 6.6 months (95% CI: 4.1–11.6 months) in the control arm (HR 0.36, 95% CI: 0.21–0.62), including both the double-blind and the open-label periods, with patients underwent cross over.
Safety of ripretinib was in harmony with prior knowledge: the most common grade 1–2 drug-related adverse events (AEs) were alopecia (49%), myalgia (28%), nausea (26%), fatigue (26%), palmar-plantar erythrodysesthesia (21%) and diarrhea (20%).
Most usual grade 3–4 AEs in the experimental arm were instead lipase increase (5%), hypertension (4%), hypophosphatemia (2%) and fatigue (2%).
Among the most severe drug-related AEs it is worth noticing a single event of cardiac failure and upper gastrointestinal hemorrhage. Six percent of patients receiving ripretinib had to reduce dosage and only 5% had to definitively discontinue the drug for treatment-related AEs.
After INVICTUS, INTRIGUE (NCT03673501) (46,47), a randomized, open-label, phase III trial is enrolling patients to investigate the efficacy of ripretinib compared to sunitinib as second-line therapy following imatinib in patients with advanced GIST. About 358 patients will be randomly assigned in a 1:1 ratio to receive either ripretinib 150 mg daily continuous on 42-day cycles or sunitinib 50 mg daily for 4 weeks with 2-week pause on 42-day cycles.
The primary endpoint of INTRIGUE is PFS as assessed by blinded independent central review (BICR), while secondary endpoints are OS and ORR (assessed by BICR).
In China an open-label, multicentre, phase II trial (NCT04282980) is recruiting patients to evaluate safety, efficacy and pharmacokinetics of ripretinib in approximately 35 patients with advanced GIST whit progressive disease after previous treatments. The primary endpoint is PFS based on independent imaging review and secondary endpoints are ORR and OS.
In USA on 15 May 2020 ripretinib was approved by the Food and Drug Administration (FDA) for adult patients with advanced GIST who were treated with ≥3 kinase inhibitors, including imatinib (48).
In Europe a Marketing Authorisation Application for Ripretinib has been submitted to the European Medicines Agency (EMA) and an Expanded Access Program (EAP) is still available for the supply of ripretinib for patients receiving the drug (49).
Avapritinib
Avapritinib (formerly known as BLU-285) is a novel, strong and selective type I inhibitor with activity against KIT and PDGFRA activation loop mutations, including PDGFRA exon 18 (D842V) and KIT exon 11, exons 11/17 and exon 17 (D816V).
Both in vitro and in vivo preclinical studies avapritinib revealed a robust activity in GISTs harboring different KIT and PDGFRA mutations with dissimilar sensitivity to traditional TKIs, resulting in reduction of tumor volume, suppression of proliferation, amplifying apoptosis and sometimes in conspicuous histologic responses (50,51).
Regarding PDGFRA, some mutations are proved to be sensitive to imatinib, for example V561D or deletion DIMH842-845, whereas other alterations, like PDGFRA D842V, PDGFRA D842Y, or PDGFRA DI842-843IM, are related with treatment refractory in vitro.
The most typical PDGFRA mutation is the D842V which arises in the exon 18 that codifies for the activation loop, causing resistance to other type 2 TKIs that usually bind to the inactive conformation (50,52).
In fact, before avapritinib approval, patients with advanced gastrointestinal stromal tumours carrying the D842V mutation had a prognosis comparable to those in pre-imatinib era (53).
NAVIGATOR (NCT02508532) (54) was the first prospective trial testing the use of avapritinib in D842V mutated gastrointestinal stromal tumours, evaluating ORR, safety and duration of response (DOR) as crucial endpoints.
It was a multicenter, open-label, phase I trial with two phases: a dose-escalation phase for patients with unresectable GIST and a subsequent dose expansion phase for patients with unresectable PDGFRA D842V-mutant GIST despite previous therapy or GIST with other mutations and progressive disease after treatment with imatinib or more TKIs.
At the time of the data cut-off, 46 patients were enrolled in the dose-escalation phase, 20 of them had a PDGFRA D842V-mutant GIST; following that, 36 patients with a PDGFRA D842V-mutant GIST were included in the dose-expansion phase.
The maximum tolerable dose was 400 mg, with a 300 mg oral dose advised for phase II.
Avapritinib had excellent anticancer activity, with an ORR of 88% (95% CI: 76–95%) in the PDGFRA exon 18 mutant group, with 9% full response and 79% PR. At 12 months, the response duration was 70% (95% CI: 54–87%), and PFS was 81% (95% CI: 69–93%).
The trial also revealed that the drug had a tolerable safety profile: the majority of treatment-related AEs were grade 1–2, with the 400-mg cohort having a greater incidence of commonly reported AEs than the 300-mg group. The most common side effects with the 300 mg dose were nausea (69%), diarrhea (41%), hyporexia (38%), and exhaustion (38%), while the most common side effects with the 400 mg dose were nausea (71%), vomiting (47%), and periorbital edema (38%). Anemia was the most common grade 3–4 drug-related side event (17%).
Conversely, toxicities associated with other anti-angiogenic TKI like hypertension and hand-foot skin reactions were infrequent.
Among AEs it is worth to mention intracranial bleeding (2%) and cognitive effects such as memory impairment (30%), cognitive disorder (10%), confusional state (9%) and encephalopathy (2%): those effects were generally grade 1, more frequent at 400 mg and showed improvement after discontinuation or dose reduction, although the most common reasons for treatment suspension were disease progression (32%). No treatment-related deaths were registered.
VOYAGER (NCT03465722) (55,56) is an open-label phase III study randomizing patients previously treated with imatinib or 1 or 2 other TKIs with locally advanced unresectable or metastatic GIST to receive either oral avapritinib (n=240) at 300 mg daily or oral regorafenib (n=236) at 160 mg daily on a 3-week-on/1-week-off schedule.
The study has missed the primary end point with avapritinib showing a mPFS of 4.2 months compared to 5.6 months for regorafenib, with a non-stat significant difference between groups.
Secondary endpoints were ORR, OS, and quality of life. The overall response rate was 17% for the experimental arm and 7% for the control group.
However, avapritinib was basically well-tolerated with most AEs described as grade 1 or 2, in line with previously reported data.
The results of the VOYAGER study are currently being analyzed.
The FDA approved avapritinib (AYVAKITTM, Blueprint Medicines Corporation) for adults with unresectable or metastatic GIST with a PDGFRA exon 18 mutation, such as the D842V mutation, on January 9, 2020, and the EMA approved it on September 24, 2020 (57,58).
In the United States, the TKI is being evaluated as a 4th-line treatment for GIST, while in the EU, it is being evaluated for the treatment of PDGFRA D842V GIST, regardless of previous therapy (59).
Other investigational drugs
Sorafenib
Sorafenib is an oral multikinase inhibitor that blocks RAF kinase and VEGFR2 and 3, as well as PDGFRB, KIT, FLT-3 and RET, resulting in antiproliferative and antiangiogenic properties. In a prospective, multicenter, phase II trial, sorafenib demonstrated activity in unresectable, KIT mutated, imatinib and sunitinib resistant GIST. In this trial were reported a PFS of 5.2 months and a disease control rate (DCR) of 68%, with 13% of patients obtaining a PR and 55% a SD (60). Other multicenter studies confirm the efficacy of sorafenib to achieve long-term tumor control. In a retrospective analysis conducted on 124 patients who progressed on imatinib and sunitinib, sorafenib demonstrated a DCR of 67% and a mPFS of 6.4 months (61). Similarly, a Korean study on 31 patients progressing after imatinib and sunitinib treatment showed a mPFS of 4.9 months. All these data were obtained in the pre-regorafenib era and, to the best of our knowledge, no further clinical trials were conducted endorsing the use of sorafenib in GIST pre-treated patients. Despite the encouraging results described, neither FDA or EMA approved the use of sorafenib in GIST patients, even if it may be prescribed off-label for this indication.
Nilotinib
Nilotinib is a selective TKI targeting BCR-ABL, PDGFRA, PDGFRB, KIT, ABL1, DDR-1 and DDR-2. This drug was investigated as third-line therapy in a phase III study in 248 patients who were resistant or intolerant to imatinib or sunitinib. mPFS, which was the primary endpoint of the trial, was not superior in the investigational arm if compared to best supportive care (BSC) (111 vs. 109 days, HR 0.90, P=0.56). Anyway, a post-hoc subset analysis revealed that, in patients who received only one prior therapy, OS was longer in favor of nilotinib (405 vs. 280 days) (62). Efficacy of nilotinib was investigated also in the first-line setting in a phase III trial (ENESTg1), compared with imatinib. Although the tolerability profile of nilotinib was similar to imatinib, the study did not meet its primary endpoint, with a 2-year PFS higher in the imatinib arm (59.2% vs. 51.6%) (63). As for sorafenib, nilotinib is mentioned in the National Comprehensive Cancer Network (NCCN) GIST treatment guidelines for possible off-label use in patients with imatinib and sunitinib resistant disease.
Pazopanib
Pazopanib is a multitargeted TKI which inhibits KIT, PDGFR, and is particularly active against VEGFR. Pazopanib has been studied in 25 patients after failure of imatinib and sunitinib, in a phase II multicenter trial. The 24-week non-progression (CR + PR + SD), was 17% with SD observed in 48% of patients. The study included one patient with SDH-deficient GIST, who exhibited prolonged disease control after 17 months (64). In 2016 were published on Lancet the results of the PAZOGIST, a phase II trial comparing pazopanib plus BSC vs. BSC alone in patients with imatinib and sunitinib resistant GIST. mPFS was longer in the investigational arm (3.4 vs. 2.3 months; 95% CI: 0.37–0.96, P=0.03). It should be noted that study patients did not receive regorafenib, that is the approved third-line therapy in refractory GIST. Despite the modest improvement in PFS, this study contributed to adding another useful agent to the existing TKI arsenal. Anyway, FDA or EMA have still not approved pazopanib for treatment of GIST (65). These results were confirmed by the recent PAGIST trial, a phase II multicenter trial evaluating safety and efficacy of pazopanib in 72 patients with locally advanced or progressive metastatic GIST. The mPFS was 19.6 weeks (95% CI: 12.6–23.4 weeks), similar to the results observed in the GRID trial with regorafenib (66).
Cabozantinib
Cabozantinib is a novel compound targeting MET, RET, KIT, VEGFR, AXL. This small molecule has proven to be effective in both imatinib-sensitive and resistant models. This effect is believed to be related to the dual KIT and MET inhibition: in fact, the upregulation of MET signaling seems to be the result of imatinib inhibition of the KIT pathway (67). The European Organization for Treatment of Cancer (EORTC) conducted the CaboGIST trial, a multicenter, open-label, phase II study, assessing the activity and safety of cabozantinib after progression with imatinib and sunitinib, including a total of 50 patients. This trial met its primary endpoint with 60% of patients (30/50) being progression-free at 12 weeks. mPFS was 5.5 months (95% CI: 3.6–6.0 months) and median OS was 18.2 months (95% CI: 14.3–22.3 months). Clinical benefit (CR, PR and SD) was observed in patients with different mutational status, including KIT exons 11, 9, 13, 14 and 17 and also in NF1- and RBPMS-NTRK3 driven GIST (68).
FGFR inhibitors
Recently, FGF activation signaling has been identified as an alternative mechanism promoting imatinib resistance in KIT/PDGFRA mutant GIST (69). Some authors described the existence of a crosstalk between FGFR and KIT illustrating how FGF2 silencing restores imatinib response in resistant GIST cells line (70). Moreover, the combination of imatinib with BGJ398, a selective FGFR inhibitor, resulted in an impressive effect on tumor growth in vivo (71), despite the following phase Ib study was early interrupted due to unacceptable toxicity (72). Therefore, FGF/FGFR events, as receptor gene mutation or fusion and ligand overexpression have been described as possible oncogenic mechanisms in SDH-deficient and quadruple WT GIST (69). These observations led to clinical investigation of multi-target compounds, active against FGF/FGFR signaling, beside Regorafenib, which has known FGFR inhibiting activity.
Dovitinib
Dovitinib, a multikinase inhibitor targeting FGFR1/2/3 has been investigated in the multicenter, prospective, phase II DOVIGIST study as second line therapy in GIST patients after imatinib failure. Among 39 patients enrolled, the ORR (CR + PR) and the DCR (CR + PR + SD), were 5.6% and 60.5% respectively at the end of the study, while mPFS was 4.6 months (90% CI: 2.8–7.4 months). Despite the study included patients with different KIT and PDGFRA baseline mutations, the small sample size precluded the efficacy of dovitinib according to GIST mutational status (73).
Masitinib
Masitinib was tested in both first- and second-line settings. A phase II clinical trial of masitinib was conducted in 30 imatinib naïve patients. At 2 months, response rate was 20% according to RECIST 1.1 criteria and 86% according to FDG-PET response criteria. The estimated PFS was 41.3 months (74). As mentioned, masitinib was also investigated in a multicenter, prospective, randomized phase II trial vs. sunitinib, after imatinib failure. Forty-four patients were randomized to receive masitinib or sunitinib (1:1). The masitinib group had a mPFS of 3.7 months after 14 months, while the control group had a mPFS of 1.9 months. The median OS in the masitinib group was not reached (expected to be 21.2 months), but it was 15.2 months in the sunitinib group. Researchers did a follow-up analysis at 26 months to see if the OS improvement had been sustained over time. Results showed median OS was 29.8 months in the investigational arm and 17.4 months in the sunitinib arm. Moreover, masitinib showed a better safety profile than sunitinib (75).
Ponatinib
Ponatinib, another multi-TKI inhibitor, with activity against a broad spectrum of mutant isoforms of KIT, including secondary exon 17 resistance mutants. The phase II multicenter POETIG trial evaluated safety and efficacy of lower dose of ponatinib in pretreated patients with KIT mutant GIST. mPFS was 86 days with single patients experiencing long lasting responses (75% quartile 210 days, maximum 420 days) (76). Despite the well0known inhibitory activity against KIT mutations, some studies highlighted the ponatinib effect also in FGFR amplified or FGFR mutated cancer cell lines (77).
Lenvatinib
Lenvatinib is a multikinase inhibitor active against FGFR, KIT, PDGFRA, RET and VEGFR. Efficacy and safety of lenvatinib in patients with GIST after failure of imatinib and sunitinib, is still under evaluation in the multicenter, randomized, placebo-controlled phase II trial LENVAGIST. Recruitment started in January 2020 and its completion is expected for March 2023 (78).
A number of other multitarget TKI have been investigated in the setting of patients with pretreated metastatic GIST, showing promising results in small phase II trials. Beyond the compounds previously mentioned, other drugs examined include dasatinib, vatalanib, and linsitinib (79-81).
PDGFRA D842V mutant GIST
Crenolanib
Crenolanib is a selective inhibitor of FTL3 and PDGFRA, and the first TKI with encouraging activity against PDGFRA D842V mutant GIST. Its efficacy in GIST patients was tested in a phase II trial (82) and is currently being assessed in 120 patients with D842V mutant GIST in the CrenoGIST trial, a randomized, double blind, placebo-controlled phase III study (83).
Target therapies beyond KIT and PDGFRA and combination treatments
Secondary resistance emerging from TKI exposure is one of the most critical problems in the treatment of GIST, with each TKI having its own resistance profile. The discovery of novel chemical agents targeting dysregulated downstream signaling pathways beyond the recognized oncogenic driver has resulted from breakthroughs in understanding the molecular basis of GIST.
PI3K/AKT/mTOR pathway
KIT and PDGFRA mutations have an impact on the activation of the PI3K/AKT/mTOR signaling which has a critical effect on cell proliferation, apoptosis, differentiation and metabolism. Some clinical trials explored this pathway as a promising target therapy strategy for GIST treatment (84).
A phase II study explored efficacy and safety of imatinib, given at 600 mg daily, combined with 2.5 mg/day everolimus. Seventy-five patients were enrolled and stratified in two groups, according to progression after imatinib only or imatinib and sunitinib/other TKI. mPFS was 1.9 months in the first group and 3.5 months in the second one. Median OS was 14.9 and 10.7 months, respectively. The combination treatment was well tolerated in the treated population (85).
Other PI3K/mTOR inhibitors have been studied, showing promising effects against both imatinib resistant and sensitive xenograft models, but further investigations are needed (86).
ETS variant transcription factor 1 (ETV1)
The ETV1 has been shown to be involved in growth and survival of interstitial cells of Cajal and GIST and represents a key downstream effector of KIT. In GIST, the constitutive activation of KIT and its downstream MAPK, prolongs ETV1 protein stability, promoting tumorigenesis. Some studies demonstrated that the concomitant inhibition of KIT and MAPK destabilizes ETV1, resulting in cytotoxic effects (87). The combination of imatinib (400 mg daily) and the MAPK inhibitor binimetinib (30 mg twice daily) was evaluated in a single arm phase II trial which enrolled 39 patients with untreated advanced GIST. The study met its primary endpoint showing an overall response rate of 68.4% and a resectability conversion rate (RCR) of 88.9%. The combination treatment showed a manageable toxicity and considering the promising results, further investigation in comparison with imatinib as frontline treatment is needed (88).
Heat shock protein 90 (HSP90)
HSP90 regulates conformation, function and activation of a number of client proteins including KIT. Inhibition of HSP90 has been explored as a novel strategy for treatment of GIST. A phase II Japanese trial investigated the efficacy of pimitespib (also known as TAS-116), an orally selective HSP90 inhibitor in 41 patients with advanced GIST after failure of imatinib, sunitinib and regorafenib. mPFS and OS were 4.4 and 11.5 months respectively, with 85% of patients achieving SD for more than 6 weeks (89).
Histone deacetylase
Histone acetylation and deacetylation are critical epigenetic mechanisms regulating gene expression and transcription. Histone deacetylase also targets multiple nonhistone substrates involved in cell proliferation, metastasis and invasion, such as α-tubulin, cortactin or HSP90. A phase I trial evaluated the activity of panobinostat, a histone deacetylase inhibitor, in combination with imatinib in extensively pretreated GIST patients. One of the 11 patients recruited had metabolic PR, seven had metabolic stability for more than 3 weeks, and three had advanced. Treatment lasted a total of 17 weeks, with a median of 6 weeks (90).
Insulin-like growth factor 1
Insulin-like growth factor 1 receptor is overexpressed in KIT/PDGFR WT GIST, particularly in those with SDH-deficiency, contributing to an increased growth signaling. A phase II study investigated the efficacy of linsitinib, a IGFR1 inhibitor in patients with WT GIST. While no objective responses were observed, metabolic PR and SD were seen in 12% and 65% of patients respectively. Clinical benefit rate (CR, PR and SD ≥9 months) at 9 months was 40%, while PFS and OS estimates at 9 months were 52% and 80% respectively (81).
Neurotrophic tropomyosin receptor kinase (NTRK)
NTRK chromosomal aberrations are observed in several tumor types, resulting in constitutive activation and aberrant expression of tropomyosin receptor kinase (TRK) kinases. NTRK gene fusions are uncommon in GIST and should be checked in patients with quadruple WT GIST (lacking KIT, PDGFRA, BRAF, SDH mutations). The oral TRK inhibitor larotrectinib was found to be effective in 17 different tumor types. In the larotrectinib data set, 71 tumors treated (47%) were sarcomas, with 4 of them (6%), being GIST. In adult and pediatric patients with sarcoma harboring an NTRK fusion, the ORR with larotrectinib was 74% and 94%, respectively. The median length of response, PFS, and OS at 15.6, 13.0, and 14.1 months, respectively, were not estimable, 28.3, and 44.4 months. A minor number of sarcoma patients (n=13), including GIST, were included in the overall entrectinib clinical trial dataset. The ORR was 46% in the sarcoma subset, while median DOR, PFS and OS were 10.3, 11.0 and 16.8 months, respectively. Larotrectinib is approved by FDA and EMA, while entrectinib only by FDA, for treatment of adults and pediatric patients with advanced solid tumors harboring NTRK gene fusions (91,92).
Immunotherapy
In the last few years, several investigations explored the role of GIST immune microenvironment. Some researchers shown how tumor-infiltrating immune cells play an important role in tumor surveillance and are linked to disease outcome, as well as enhancing imatinib’s anticancer activity. In patients with advanced GIST who had had at least imatinib, a phase II trial looked at the efficacy of nivolumab with or without ipilimumab. In the nivolumab arm, SD was the best response in 7 of the 15 patients. In the combo arm, 1 out of every 12 patients had PR and 2 out of every 12 had SD. The nivolumab alone arm had a mPFS of 12.1 weeks and the doublet checkpoint inhibition arm had a mPFS of 8.3 weeks (93). Currently, several clinical trials are ongoing with the aim to explore the activity of immune checkpoint inhibitors alone or in combination with similar agents or with TKI.
Conclusions
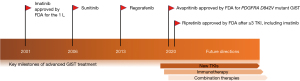
GIST treatment and prognosis have been revolutionized in 2001 by the advent of imatinib, which is still the first line treatment of choice for the majority of patients with advanced disease. Since then, other two TKI, have been approved for GIST treatment, with tangible improvement in patient outcome in a disease previously deemed as resistant to systemic therapy. Despite this substantial survival benefit, secondary resistance mutations to current drugs and their heterogeneity, remains a major challenge in GIST management. Advances in our understanding of GIST biology have facilitated the development of various novel therapeutic options with the aim to overcome this issue. Recently the armamentarium for treatment of GIST has been enriched by ripretinib, active against multiple resistance mutations, and avapritinib effective in PDGFRA D842V GIST. Several clinical trials testing other promising compounds have been designed and should be supported in order to improve patients’ outcomes. Future arduous challenges will be to personalize GIST treatment through the identification of predictive factors of response to target drugs, the systematic characterization of resistance mechanism (with liquid biopsy potentially representing an ideal ally) and to better understand the optimal way to sequence and combine treatments. The ability to tailor GIST treatment to the characteristics of each patient will help to maximize the benefits of these targeted therapies, with the aim to improve patient prognosis Figure 2.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Bernd Kasper and Eva Wardelmann) for the series “Gastrointestinal Stromal Tumors” published in Gastrointestinal Stromal Tumor. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://gist.amegroups.com/article/view/10.21037/gist-21-6/rc
Peer Review File: Available at https://gist.amegroups.com/article/view/10.21037/gist-21-6/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gist.amegroups.com/article/view/10.21037/gist-21-6/coif). The series “Gastrointestinal Stromal Tumors” was commissioned by the editorial office without any funding or sponsorship. AM reports grants from Deciphera, Eli Lilli, Pharmamar and Molteni. FP reports grants from Pharmamar and Molteni, outside the submitted work. AN reports grants from Eli Lilli, Pharmamar and Molteni, and personal fees from Eli Lilli, outside the submitted work. MS reports personal fees from Eisai and Bayer, outside the submitted work. BV reports personal fees from Eisai, Eli Lilli, Pharmamar, Abbott, Novartis, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Miettinen M, Lasota J. Histopathology of gastrointestinal stromal tumor. J Surg Oncol 2011;104:865-73. [Crossref] [PubMed]
- Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer 2011;11:865-78. [Crossref] [PubMed]
- Joensuu H, Vehtari A, Riihimäki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol 2012;13:265-74. [Crossref] [PubMed]
- Ducimetière F, Lurkin A, Ranchère-Vince D, et al. Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PLoS One 2011;6:e20294. [Crossref] [PubMed]
- Matyakhina L, Bei TA, McWhinney SR, et al. Genetics of carney triad: recurrent losses at chromosome 1 but lack of germline mutations in genes associated with paragangliomas and gastrointestinal stromal tumors. J Clin Endocrinol Metab 2007;92:2938-43. [Crossref] [PubMed]
- Carney JA, Stratakis CA. Familial paraganglioma and gastric stromal sarcoma: a new syndrome distinct from the Carney triad. Am J Med Genet 2002;108:132-9. [Crossref] [PubMed]
- Yantiss RK, Rosenberg AE, Sarran L, et al. Multiple gastrointestinal stromal tumors in type I neurofibromatosis: a pathologic and molecular study. Mod Pathol 2005;18:475-84. [Crossref] [PubMed]
- Kindblom LG, Remotti HE, Aldenborg F, et al. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol 1998;152:1259-69. [PubMed]
- Sarlomo-Rikala M, Kovatich AJ, Barusevicius A, et al. CD117: a sensitive marker for gastrointestinal stromal tumors that is more specific than CD34. Mod Pathol 1998;11:728-34. [PubMed]
- West RB, Corless CL, Chen X, et al. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am J Pathol 2004;165:107-13. [Crossref] [PubMed]
- Medeiros F, Corless CL, Duensing A, et al. KIT-negative gastrointestinal stromal tumors: proof of concept and therapeutic implications. Am J Surg Pathol 2004;28:889-94. [Crossref] [PubMed]
- Lux ML, Rubin BP, Biase TL, et al. KIT extracellular and kinase domain mutations in gastrointestinal stromal tumors. Am J Pathol 2000;156:791-5. [Crossref] [PubMed]
- Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science 2003;299:708-10. [Crossref] [PubMed]
- Blume-Jensen P, Claesson-Welsh L, Siegbahn A, et al. Activation of the human c-kit product by ligand-induced dimerization mediates circular actin reorganization and chemotaxis. EMBO J 1991;10:4121-8. [Crossref] [PubMed]
- Yuzawa S, Opatowsky Y, Zhang Z, et al. Structural basis for activation of the receptor tyrosine kinase KIT by stem cell factor. Cell 2007;130:323-34. [Crossref] [PubMed]
- Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet 2013;382:973-83. [Crossref] [PubMed]
- Kinoshita K, Hirota S, Isozaki K, et al. Absence of c-kit gene mutations in gastrointestinal stromal tumours from neurofibromatosis type 1 patients. J Pathol 2004;202:80-5. [Crossref] [PubMed]
- Miranda C, Nucifora M, Molinari F, et al. KRAS and BRAF mutations predict primary resistance to imatinib in gastrointestinal stromal tumors. Clin Cancer Res 2012;18:1769-76. [Crossref] [PubMed]
- Joensuu H, Rutkowski P, Nishida T, et al. KIT and PDGFRA mutations and the risk of GI stromal tumor recurrence. J Clin Oncol 2015;33:634-42. [Crossref] [PubMed]
- George S, Jones RL, Bauer S, et al. Avapritinib in Patients With Advanced Gastrointestinal Stromal Tumors Following at Least Three Prior Lines of Therapy. Oncologist 2021;26:e639-49. [Crossref] [PubMed]
- Edmonson JH, Marks RS, Buckner JC, et al. Contrast of response to dacarbazine, mitomycin, doxorubicin, and cisplatin (DMAP) plus GM-CSF between patients with advanced malignant gastrointestinal stromal tumors and patients with other advanced leiomyosarcomas. Cancer Invest 2002;20:605-12. [Crossref] [PubMed]
- van Oosterom AT, Judson I, Verweij J, et al. Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours: a phase I study. Lancet 2001;358:1421-3. [Crossref] [PubMed]
- Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002;347:472-80. [Crossref] [PubMed]
- Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST). Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients. J Clin Oncol 2010;28:1247-53. [Crossref] [PubMed]
- Debiec-Rychter M, Sciot R, Le Cesne A, et al. KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer 2006;42:1093-103. [Crossref] [PubMed]
- Patel S, Zalcberg JR. Optimizing the dose of imatinib for treatment of gastrointestinal stromal tumours: lessons from the phase 3 trials. Eur J Cancer 2008;44:501-9. [Crossref] [PubMed]
- Hislop J, Mowatt G, Sharma P, et al. Systematic review of escalated imatinib doses compared with sunitinib or best supportive care, for the treatment of people with unresectable/metastatic gastrointestinal stromal tumours whose disease has progressed on the standard imatinib dose. J Gastrointest Cancer 2012;168-76. [Crossref] [PubMed]
- Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol 2008;26:620-5. [Crossref] [PubMed]
- Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 2006;368:1329-38. [Crossref] [PubMed]
- Heinrich MC, Maki RG, Corless CL, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J Clin Oncol 2008;26:5352-9. [Crossref] [PubMed]
- Demetri GD, Heinrich MC, Fletcher JA, et al. Molecular target modulation, imaging, and clinical evaluation of gastrointestinal stromal tumor patients treated with sunitinib malate after imatinib failure. Clin Cancer Res 2009;15:5902-9. [Crossref] [PubMed]
- Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:295-302. [Crossref] [PubMed]
- Serrano C, García-Del-Muro X, Valverde C, et al. Clinicopathological and Molecular Characterization of Metastatic Gastrointestinal Stromal Tumors with Prolonged Benefit to Frontline Imatinib. Oncologist 2019;24:680-7. [Crossref] [PubMed]
- Antonescu CR, Besmer P, Guo T, et al. Acquired resistance to imatinib in gastrointestinal stromal tumor occurs through secondary gene mutation. Clin Cancer Res 2005;11:4182-90. [Crossref] [PubMed]
- Liegl B, Kepten I, Le C, et al. Heterogeneity of kinase inhibitor resistance mechanisms in GIST. J Pathol 2008;216:64-74. [Crossref] [PubMed]
- Serrano C, Mariño-Enríquez A, Tao DL, et al. Complementary activity of tyrosine kinase inhibitors against secondary kit mutations in imatinib-resistant gastrointestinal stromal tumours. Br J Cancer 2019;120:612-20. [Crossref] [PubMed]
- Serrano C, Vivancos A, López-Pousa A, et al. Clinical value of next generation sequencing of plasma cell-free DNA in gastrointestinal stromal tumors. BMC Cancer 2020;20:99. [Crossref] [PubMed]
- Antonescu CR, DeMatteo RP. CCR 20th Anniversary Commentary: A Genetic Mechanism of Imatinib Resistance in Gastrointestinal Stromal Tumor-Where Are We a Decade Later? Clin Cancer Res 2015;21:3363-5. [Crossref] [PubMed]
- Gounder MM, Maki RG. Molecular basis for primary and secondary tyrosine kinase inhibitor resistance in gastrointestinal stromal tumor. Cancer Chemother Pharmacol 2011;67:S25-43. [Crossref] [PubMed]
- Lostes-Bardaji MJ, García-Illescas D, Valverde C, et al. Ripretinib in gastrointestinal stromal tumor: the long-awaited step forward. Ther Adv Med Oncol 2021;13:1758835920986498. [Crossref] [PubMed]
- Farag S, Smith MJ, Fotiadis N, et al. Revolutions in treatment options in gastrointestinal stromal tumours (GISTs): the latest updates Curr Treat Options Oncol 2020;21:55. [Crossref] [PubMed]
- Smith BD, Kaufman MD, Lu WP, et al. Ripretinib (DCC-2618) Is a Switch Control Kinase Inhibitor of a Broad Spectrum of Oncogenic and Drug-Resistant KIT and PDGFRA Variants. Cancer Cell 2019;35:738-751.e9. [Crossref] [PubMed]
- Schneeweiss M, Peter B, Bibi S, et al. The KIT and PDGFRA switch-control inhibitor DCC-2618 blocks growth and survival of multiple neoplastic cell types in advanced mastocytosis. Haematologica 2018;103:799-809. [Crossref] [PubMed]
- von Mehren M, Serrano C, Bauer S, et al. LBA87 - INVICTUS: A phase III, interventional, double-blind, placebo-controlled study to assess the safety and efficacy of ripretinib as ≥ 4th-line therapy in patients with advanced gastrointestinal stromal tumors (GIST) who have received treatment with prior anticancer therapies (NCT03353753). Ann Oncol 2019;30:v925-6. [Crossref]
- Janku F, Abdul Razak AR, Chi P, et al. Switch Control Inhibition of KIT and PDGFRA in Patients With Advanced Gastrointestinal Stromal Tumor: A Phase I Study of Ripretinib. J Clin Oncol 2020;38:3294-303. [Crossref] [PubMed]
- Nemunaitis J, Bauer S, Blay JY, et al. Intrigue: Phase III study of ripretinib versus sunitinib in advanced gastrointestinal stromal tumor after imatinib. Future Oncol 2020;16:4251-64. [Crossref] [PubMed]
- Dhillon S. Ripretinib: First Approval. Drugs 2020;80:1133-8. [Crossref] [PubMed]
- FDA approves ripretinib for advanced gastrointestinal stromal tumor. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ripretinib-advanced-gastrointestinal-stromal-tumor
- Deciphera Pharmaceuticals. Our pipeline: expanded access. 2020. Available online: https://www.deciphera.com/pipeline/expanded-access/. Accessed 11 Jun 2020.
- Evans EK, Gardino AK, Kim JL, et al. A precision therapy against cancers driven by KIT/PDGFRA mutations. Sci Transl Med 2017;9:eaao1690.
- Gebreyohannes YK, Wozniak A, Zhai ME, et al. Robust Activity of Avapritinib, Potent and Highly Selective Inhibitor of Mutated KIT, in Patient-derived Xenograft Models of Gastrointestinal Stromal Tumors. Clin Cancer Res 2019;25:609-18. [Crossref] [PubMed]
- Indio V, Astolfi A, Tarantino G, et al. Integrated Molecular Characterization of Gastrointestinal Stromal Tumors (GIST) Harboring the Rare D842V Mutation in PDGFRA Gene. Int J Mol Sci 2018;19:732. [Crossref] [PubMed]
- Cassier PA, Fumagalli E, Rutkowski P, et al. Outcome of patients with platelet-derived growth factor receptor alpha-mutated gastrointestinal stromal tumors in the tyrosine kinase inhibitor era. Clin Cancer Res 2012;18:4458-64. [Crossref] [PubMed]
- Heinrich MC, Jones RL, von Mehren M, et al. Avapritinib in advanced PDGFRA D842V-mutant gastrointestinal stromal tumour (NAVIGATOR): a multicentre, open-label, phase 1 trial. Lancet Oncol 2020;21:935-46. [Crossref] [PubMed]
- (VOYAGER) Study of Avapritinib vs Regorafenib in Patients With Locally Advanced Unresectable or Metastatic GIST. Available online: https://clinicaltrials.gov/ct2/show/NCT03465722
- Blueprint Medicines Announces Top-line Results from Phase 3 VOYAGER Trial of Avapritinib versus Regorafenib in Patients with Advanced Gastrointestinal Stromal Tumor | Blueprint Medicines Corp. Available online: https://ir.blueprintmedicines.com/news-releases/news- release-details/blueprint-medicines-announces-top-line- results-phase-3-voyager
- FDA approves avapritinib for gastrointestinal stromal tumor with a rare mutation. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-avapritinib-gastrointestinal-stromal-tumor-rare-mutation
- Ayvakyt. European Medicines Agency. Available online: https://www.ema.europa.eu/en
- Dhillon S. Avapritinib: First Approval. Drugs 2020;80:433-9. [Crossref] [PubMed]
- Campbell NP, Wroblewski K, Maki RG, et al. Final results of a University of Chicago phase II consortium trial of sorafenib (SOR) in patients (pts) with imatinib (IM)- and sunitinib (SU)-resistant (RES) gastrointestinal stromal tumors (GIST). J Clin Oncol 2011;29:abstr 4.
- Montemurro M, Gelderblom H, Bitz U, et al. Sorafenib as third- or fourth-line treatment of advanced gastrointestinal stromal tumour and pretreatment including both imatinib and sunitinib, and nilotinib: A retrospective analysis. Eur J Cancer 2013;49:1027-31. [Crossref] [PubMed]
- Park SH, Ryu MH, Ryoo BY, et al. Sorafenib in patients with metastatic gastrointestinal stromal tumors who failed two or more prior tyrosine kinase inhibitors: a phase II study of Korean gastrointestinal stromal tumors study group. Invest New Drugs 2012;30:2377-83. [Crossref] [PubMed]
- Reichardt P, Blay JY, Gelderblom H, et al. Phase III study of nilotinib versus best supportive care with or without a TKI in patients with gastrointestinal stromal tumors resistant to or intolerant of imatinib and sunitinib. Ann Oncol 2012;23:1680-7. [Crossref] [PubMed]
- Ganjoo KN, Villalobos VM, Kamaya A, et al. A multicenter phase II study of pazopanib in patients with advanced gastrointestinal stromal tumors (GIST) following failure of at least imatinib and sunitinib. Ann Oncol 2014;25:236-40. [Crossref] [PubMed]
- Mir O, Cropet C, Toulmonde M, et al. Pazopanib plus best supportive care versus best supportive care alone in advanced gastrointestinal stromal tumours resistant to imatinib and sunitinib (PAZOGIST): a randomised, multicentre, open-label phase 2 trial. Lancet Oncol 2016;17:632-41. [Crossref] [PubMed]
- Eriksson M, Reichardt P, Joensuu H, et al. Benefit of pazopanib in advanced gastrointestinal stromal tumours: results from a phase II trial (SSG XXI, PAGIST). ESMO Open 2021;6:100217. [Crossref] [PubMed]
- Cohen NA, Zeng S, Seifert AM, et al. Pharmacological Inhibition of KIT Activates MET Signaling in Gastrointestinal Stromal Tumors. Cancer Res 2015;75:2061-70. [Crossref] [PubMed]
- Schöffski P, Mir O, Kasper B, et al. Activity and safety of cabozantinib in patients with gastrointestinal stromal tumor after failure of imatinib and sunitinib: EORTC phase II trial 1317 CaboGIST. J Clin Oncol 2019;37:abstr 11006.
- Astolfi A, Pantaleo MA, Indio V, et al. The Emerging Role Of FGF/FGFR Pathway in Gastrointestinal Stromal Tumor. Int J Mol Sci 2020;21:3313. [Crossref] [PubMed]
- Javidi-Sharifi N, Traer E, Martinez J, et al. Crosstalk between KIT and FGFR3 Promotes Gastrointestinal Stromal Tumor Cell Growth and Drug Resistance. Cancer Res 2015;75:880-91. [Crossref] [PubMed]
- Boichuk S, Galembikova A, Dunaev P, et al. Targeting of FGF-Signaling Re-Sensitizes Gastrointestinal Stromal Tumors (GIST) to Imatinib In vitro and In vivo. Molecules 2018;23:2643. [Crossref] [PubMed]
- Kelly CM, Shoushtari AN, Qin LX, et al. A phase Ib study of BGJ398, a pan-FGFR kinase inhibitor in combination with imatinib in patients with advanced gastrointestinal stromal tumor. Invest New Drugs 2019;37:282-90. [Crossref] [PubMed]
- Joensuu H, Blay JY, Comandone A, et al. Dovitinib in patients with gastrointestinal stromal tumour refractory and/or intolerant to imatinib. Br J Cancer 2017;117:1278-85. [Crossref] [PubMed]
- Le Cesne A, Blay JY, Bui BN, et al. Phase II study of oral masitinib mesilate in imatinib-naïve patients with locally advanced or metastatic gastro-intestinal stromal tumour (GIST). Eur J Cancer 2010;46:1344-51. [Crossref] [PubMed]
- Adenis A, Blay JY, Bui-Nguyen B, et al. Masitinib in advanced gastrointestinal stromal tumor (GIST) after failure of imatinib: a randomized controlled open-label trial. Ann Oncol 2014;25:1762-9. [Crossref] [PubMed]
- Falkenhorst J, Hamacher R, Reichardt P, et al. Lower-dosing ponatinib in pre-treated GIST: Results of the POETIG phase II trial. J Clin Oncol 2020;38:abstr 11536.
- Gozgit JM, Wong MJ, Moran L, et al. Ponatinib (AP24534), a multitargeted pan-FGFR inhibitor with activity in multiple FGFR-amplified or mutated cancer models. Mol Cancer Ther 2012;11:690-9. [Crossref] [PubMed]
- Centre Leon Berard Multicentre Placebo-controlled Double-blinded Phase II Study of Lenvatinib Efficacy in Patients with Locally Advanced or Metastatic GIST (Gastrointestinal Stromal Tumor) After Imatinib/Sunitinib Failure (LENVAGIST) [(accessed on 9 April 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04193553
- Trent JC, Wathen K, von Mehren M, et al. A phase II study of dasatinib for patients with imatinib-resistant gastrointestinal stromal tumor (GIST). J Clin Oncol 2011;29:abtr 10006.
- Joensuu H, De Braud F, Grignagni G, et al. Vatalanib for metastatic gastrointestinal stromal tumour (GIST) resistant to imatinib: final results of a phase II study. Br J Cancer 2011;104:1686-90. [Crossref] [PubMed]
- von Mehren M, George S, Heinrich MC, et al. Linsitinib (OSI-906) for the Treatment of Adult and Pediatric Wild-Type Gastrointestinal Stromal Tumors, a SARC Phase II Study. Clin Cancer Res 2020;26:1837-45. [Crossref] [PubMed]
- von Mehren M, Tetzlaff ED, Macaraeg M, et al. Dose escalating study of crenolanib besylate in advanced GIST patients with PDGFRA D842V activating mutations. J Clin Oncol 2016;34:abstr 11010.
- Blay JY, Heinrich MC, Hohenberger P, et al. A randomized, double-blind, placebo-controlled, phase III study of crenolanib in advanced or metastatic GIST patients bearing a D842V mutation in PDGFRA: the CrenoGIST study. J Clin Oncol 2017;35:abstr TPS11080.
- Bauer S, Duensing A, Demetri GD, et al. KIT oncogenic signaling mechanisms in imatinib-resistant gastrointestinal stromal tumor: PI3-kinase/AKT is a crucial survival pathway. Oncogene 2007;26:7560-8. [Crossref] [PubMed]
- Schöffski P, Reichardt P, Blay JY, et al. A phase I-II study of everolimus (RAD001) in combination with imatinib in patients with imatinib-resistant gastrointestinal stromal tumors. Ann Oncol 2010;21:1990-8. [Crossref] [PubMed]
- Van Looy T, Wozniak A, Floris G, et al. Phosphoinositide 3-kinase inhibitors combined with imatinib in patient-derived xenograft models of gastrointestinal stromal tumors: rationale and efficacy. Clin Cancer Res 2014;20:6071-82. [Crossref] [PubMed]
- Chi P, Chen Y, Zhang L, et al. ETV1 is a lineage survival factor that cooperates with KIT in gastrointestinal stromal tumours. Nature 2010;467:849-53. [Crossref] [PubMed]
- Chi P, Qin LX, Kelly CM, et al. A phase II study of MEK162 (binimetinib [BINI]) in combination with imatinib in patients with untreated advanced gastrointestinal stromal tumor (GIST). J Clin Oncol. 2020;38:11508. [Crossref]
- Doi T, Kurokawa Y, Sawaki A, et al. Efficacy and safety of TAS-116, an oral inhibitor of heat shock protein 90, in patients with metastatic or unresectable gastrointestinal stromal tumour refractory to imatinib, sunitinib and regorafenib: a phase II, single-arm trial. Eur J Cancer 2019;121:29-39. [Crossref] [PubMed]
- Bauer S, Hilger RA, Mühlenberg T, et al. Phase I study of panobinostat and imatinib in patients with treatment-refractory metastatic gastrointestinal stromal tumors. Br J Cancer 2014;110:1155-62. [Crossref] [PubMed]
- Drilon A, Laetsch TW, Kummar S, et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med 2018;378:731-9. [Crossref] [PubMed]
- Doebele RC, Drilon A, Paz-Ares L, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol 2020;21:271-82. Erratum in: Lancet Oncol 2020;21:e70; Lancet Oncol 2020;21:e341; Lancet Oncol 2020;21:e372; Lancet Oncol 2021;22:e428. [Crossref] [PubMed]
- Singh AS, Chmielowski B, Hecht JR, et al. A randomized phase II study of nivolumab monotherapy versus nivolumab combined with ipilimumab in advanced gastrointestinal stromal tumor (GIST). J Clin Oncol 2019;37:abstr 11017.
Cite this article as: Mazzocca A, Minelli A, Paternostro F, Silletta M, Napolitano A, Vincenzi B. New molecularly targeted drugs for gist after imatinib, sunitinib and regorafenib: a narrative review. Gastrointest Stromal Tumor 2022;5:4.


